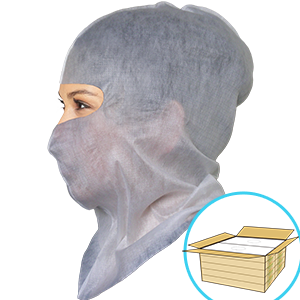
Product Overview
These masks prototypic samples for evaluation. Using these free samples exempts us (VitaFlex and its staff) from any responsibility on product performance. Shipped by USPS First Class Mail.
The main purpose of the Soft-stretch mask is to contain saliva droplets from the wearer.The masks have been tested to have a bacterial filtration efficiency (BFE) of >99.5% (ASTM F2101). However, due to wearers’ faces varying in shape and nose-bridge height, our Soft-stretch mask may not tightly cover the nose and mouth to provide the filtering protection. They should not be used as surgical masks.
“Surgical Masks” are not always higher quality. Calling disposable surgical masks “medical grade” is misleading. Registration of premarket notification 510 (k) is to notify the FDA of a company’s intention of marketing a facemask that is equivalent to an existing surgical mask in effectiveness and safety. FDA does not test to certify “Surgical Masks”; rather, they grant permission to label “Surgical Mask” based on company provided barrier properties statement.
Although the filing process is not complicated, the cost is significant. We did not file 510 (k) for our Soft-stretch Masks because we do not intend to promote them to be worn by healthcare professionals in surgical procedures.